Both Pfizer and Moderna have applied for special designations to. Interim Order Respecting the.
Covid Vaccines What Full Fda Approval Means For You
1-888-info-fda 1-888-463-6332 Contact FDA Subscribe to FDA RSS feeds Follow FDA on Twitter Follow FDA on Facebook View FDA videos on YouTube View FDA photos on Flickr.

Fda approved vaccines list. Vaxchora Product Approval. Rabies Vaccine Adsorbed. Public health experts say they see no reason why the vaccines from Pfizer-BioNTech and Moderna wont receive full approval Johnson Johnson maker of a third vaccine has yet to apply.
Two versions of AstraZeneca AZD1222. Clinical data login required Paediatric investigation plan. These are as follows.
9 Zeilen Vaccines for human use. Spikevax previously COVID-19 Vaccine Moderna Conditional marketing authorisation granted. The FDA is the regulatory authority with oversight of the safety effectiveness and quality of vaccines that are used in the US including COVID-19 vaccines.
Sinopharm SARS-CoV-2 Vaccine Vero Cell. Getting full FDA approval for a vaccine is a time consuming process that can take up to 10 months under normal circumstances. List of Vaccine Approvals.
Also see the different types of COVID-19 vaccines that currently are available or are undergoing large-scale Phase 3 clinical trials in the United States. The slide showing the FDAs draft list of possible adverse event outcomes appeared briefly during a public meeting by the US Food and Drug Administrations Product Advisory Committee on Oct 22. Full FDA approval of the COVID-19 vaccines which are now being given under an emergency use authorization EUA.
WHO has also listed the PfizerBioNTech Astrazeneca-SK Bio Serum Institute of India Janssen and Moderna vaccines for emergency use. This is the maximum review time frame. Janssen Johnson and Johnson.
The FDA has only granted emergency-use approval of the Pfizer Moderna and Johnson Johnson vaccines but the agency is expected to soon give full approval to Pfizer. Product Approval - BCG Vaccine tuberculosis BCG Package Insert. That means the FDA would decide on full approval of the Pfizer-BioNTech vaccine by January 2022 and February 2022 for the Moderna vaccine.
Poliovirus Vaccine Inactivated Monkey Kidney Cell IPOL. Viral Vector COVID-19 Vaccines. List of COVID-19 Vaccines Authorized by the FDA.
Poliovirus Vaccine Inactivated Human Diploid Cell Poliovax. Product Approval - Emergency Use Authorization EUA - COVID-19. An FDA slideshow presentation regarding Covid vaccines last year accidentally displayed a long list of possible adverse reactions to the vaccine including myocarditis seizures and even death.
FDA employees who are career. The next milestone. Vaxzevria previously COVID-19 Vaccine AstraZeneca Conditional marketing authorisation granted.
104 Zeilen 07022021. The FDA authorized the use. In fact its one of just seven vaccines that the WHO has approved for inclusion on the EUL.
EUA Full Prescribing Information. Organon Teknica Merck BCG Product Approval. The three COVID-19 vaccines produced by Pfizer-BioNTech Moderna and Johnson Johnson have been granted authorization for emergency use by the FDA in the United.
EMA recommends COVID-19 Vaccine Moderna for authorisation in the EU. Pfizer and BioNTech which developed one of the three COVID-19 vaccines available in the US in May completed their application for full FDA approval for use in. All the ones mentioned below have products that have been approved.
Product Approval - Cholera. Information about mRNA vaccines generally and COVID-19 vaccines that use this new technology specifically. Vaxchora Package Insert.
Pfizer-BioNTech COVID-19 Vaccine BNT162b2 ChAdOx1-S recombinant COVID-19 Vaccine AstraZeneca SARS-CoV-2 Vaccine Vero Cell Inactivated Coronavac Sputnik V Gam-COVID-Vac COVID-19 Vaccine. Janssen Johnson. Pfizer BNT162b2COMIRNATY Tozinameran INN Moderna mRNA-1273.
Vaccines Licensed for Use in the United States.

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021

Covid 19 Vaccine Faq Anne Arundel County Department Of Health

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021

Who And Ema Approved Vaccine List Which Covid 19 Vaccines Are On Who And Ema List Updated 18 August 2021 Wego Travel Blog

Covid 19 Vaccine Need To Know Fliers Posters And Graphics Mass Gov

Lack Of Fda Covid Vaccine Approval Doesn T Matter Office For Science And Society Mcgill University
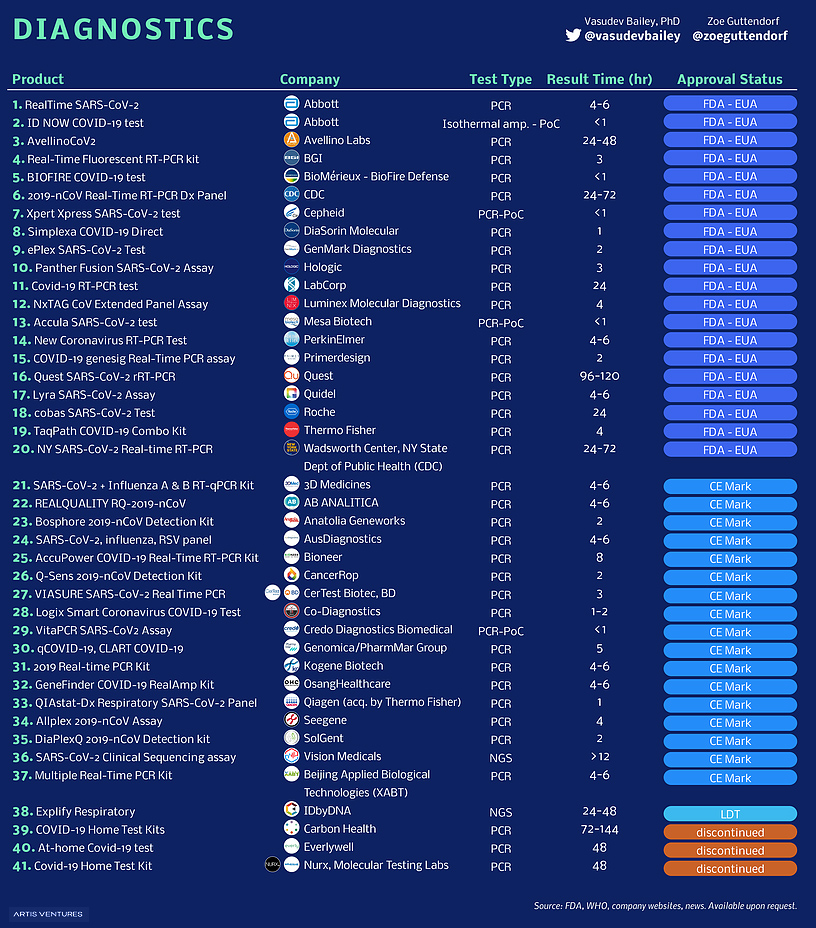
Every Vaccine And Treatment In Development For Covid 19 So Far

Covid 19 Risks And Side Effects Of Vaccination Science In Depth Reporting On Science And Technology Dw 20 01 2021

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021
Novavax Announces Further Delays For Regulatory Filings Of Covid 19 Vaccine Pmlive
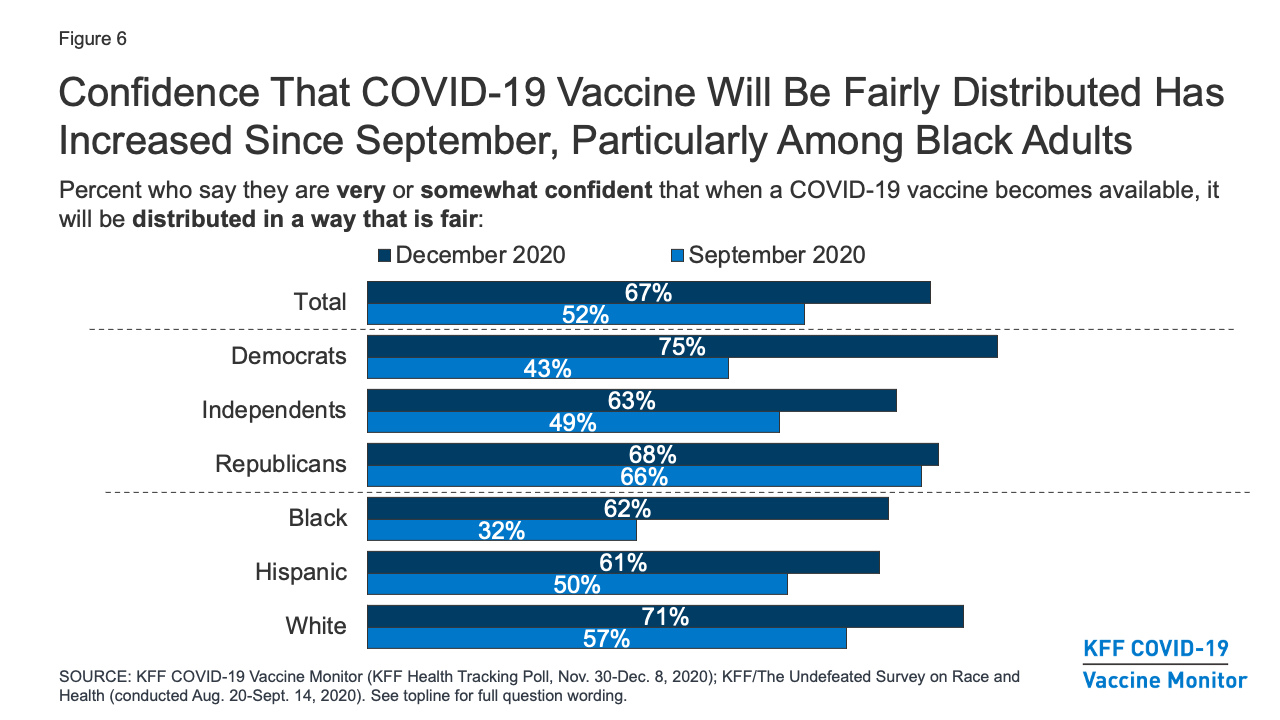
Kff Covid 19 Vaccine Monitor December 2020 Kff
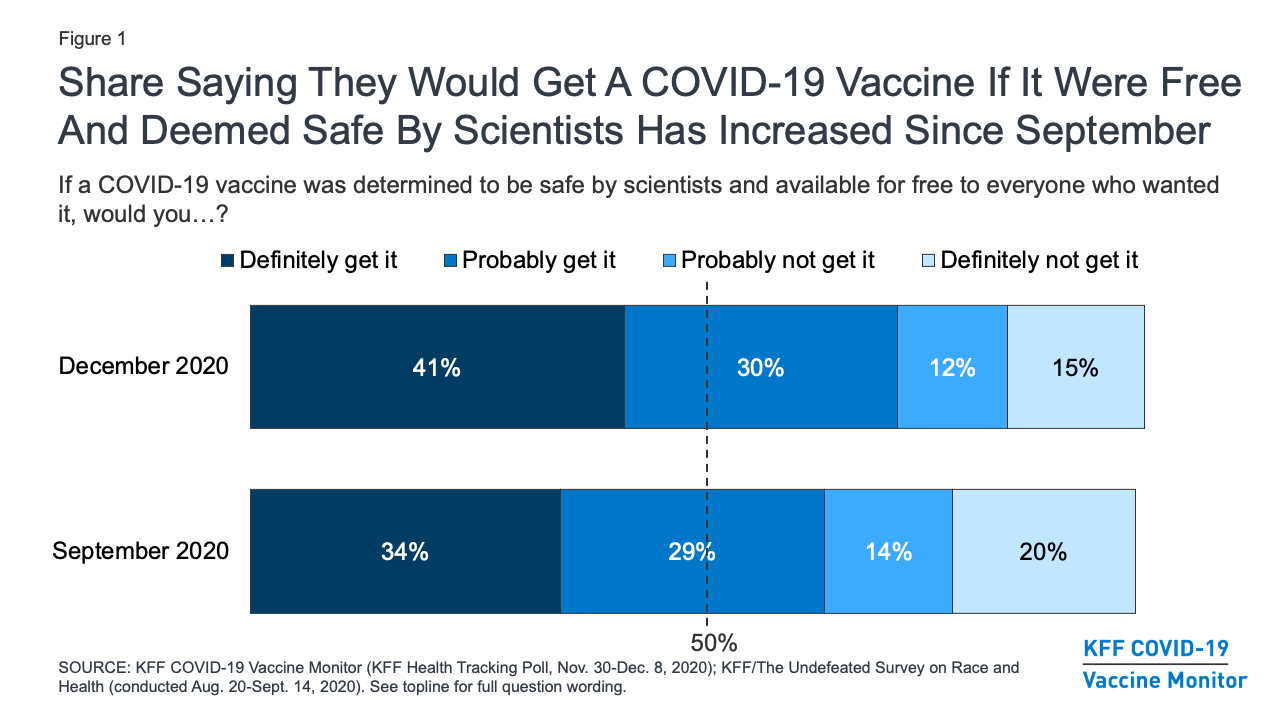
Kff Covid 19 Vaccine Monitor December 2020 Kff

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021

Know Your Vaccines Vaccine Matrix Current Evidence Department Of Health Website

Faqs Emergency Use Authorization Department Of Health Website

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021

Best Covid Vaccine To Get Comparing J J Pfizer Novavax And Moderna

Fda Advisory Panel Endorses Pfizer Biontech Covid 19 Vaccine

