The FDAs initial list of adverse reactions appeared at a public meeting of the FDAs Product Advisory Committee on the safety of COVID-19 vaccines from October 22 2020. Coronavirus Disease 2019 COVID-19 May 10 2021.

Lack Of Fda Covid Vaccine Approval Doesn T Matter Office For Science And Society Mcgill University
Pfizer-BioNTech COVID-19 Vaccine May 10 2021.
Fda approved vaccines list covid. The FDA recognizes that vaccines are key to ending the COVID-19 pandemic and is working as quickly as possible to review applications for full approval an FDA. Federal regulators are winding down the process of licensing Pfizers two-dose coronavirus vaccine setting up an approval possibly by Monday and possibly kicking off a wave of new mandates. The safety updates summarise the data that have become available since the vaccines authorisation.
The slide showing the FDAs draft list of possible adverse event outcomes appeared briefly during a public meeting by the US Food and Drug Administrations Product Advisory Committee on Oct. The FDA could grant the Pfizer-BioNTech Covid-19 vaccine full approval on Monday The New York Times reported late Friday. WHO today listed the Sinopharm COVID-19 vaccine for emergency use giving the green light for this vaccine to be rolled out globally.
As of February 27 2021 large-scale Phase 3 clinical trials are in progress or being planned for two COVID-19 vaccines in the United States. Emergency Use AuthorizationEmergency Use Authorization EUA is an authorization issued for unregistered drugs and vaccines in a public health emergency. The addition of this vaccine has the potential to rapidly accelerate COVID-19 vaccine access for countries seeking to.
COVID-19 vaccine clinical trials including vaccines in earlier stages of development by visiting clinicaltrialsgov. They also indicate whether any safety information requires further investigation. Food and Drug Administration issued an emergency use authorization EUA for the second vaccine for the prevention of coronavirus disease.
CTVNewsca examines what COVID-19 vaccines are recognized and. All the ones mentioned below have products that have been approved. Side effects accidentally shown at FDA presentation 2020 appeared for split second at 23340 of original timing of FDA.
Currently no coronavirus vaccine is fully approved. 104 rijen FDA has rigorous scientific and regulatory processes in place to facilitate development and ensure the safety effectiveness and quality of COVID-19 vaccines. WHO Certified COVID Vaccines WHO has approved Covishield which is the local name for the Oxford-AstraZeneca vaccine that is being manufactured locally by the Serum Institute of India.
121 of the President of the Philippines authorizes the issuance of the EUAList of COVID-19 Vaccines Authorized by the FDAPfizer-BioNTech COVID-19 Vaccine. The FDA Director General by virtue of the Executive Order No. An FDA slideshow presentation regarding Covid vaccines last year accidentally displayed a long list of possible adverse reactions to the vaccine including myocarditis seizures and even death.
EMA releases a monthly update for each authorised COVID-19 vaccine. In the list below the entry for each authorized drug and vaccine includes the. To see the full list of safety updates for COVID-19 vaccines click on the links below.
Sotrovimab for injection Immune sera and immunoglobulins for human use 251285 2021-07-30. The FDA is the regulatory authority with oversight of the safety effectiveness and quality of vaccines that are used in the US including COVID-19 vaccines. On December 18 2020 the US.
The FDA expanded the emergency use authorization of the Pfizer-BioNTech COVID-19 Vaccine to include adolescents 12. Its a question many are asking as coronavirus vaccinations continue across the US. 9 rijen Drugs and vaccines that have been authorized by Health Canada for use in relation to the COVID-19 pandemic are listed here.
AstraZeneca COVID-19 vaccine Novavax COVID-19 vaccine Learn more about US. The FDA expanded the emergency use authorization of the Pfizer-BioNTech COVID-19 Vaccine to include adolescents 12. Are the COVID vaccines FDA approved.
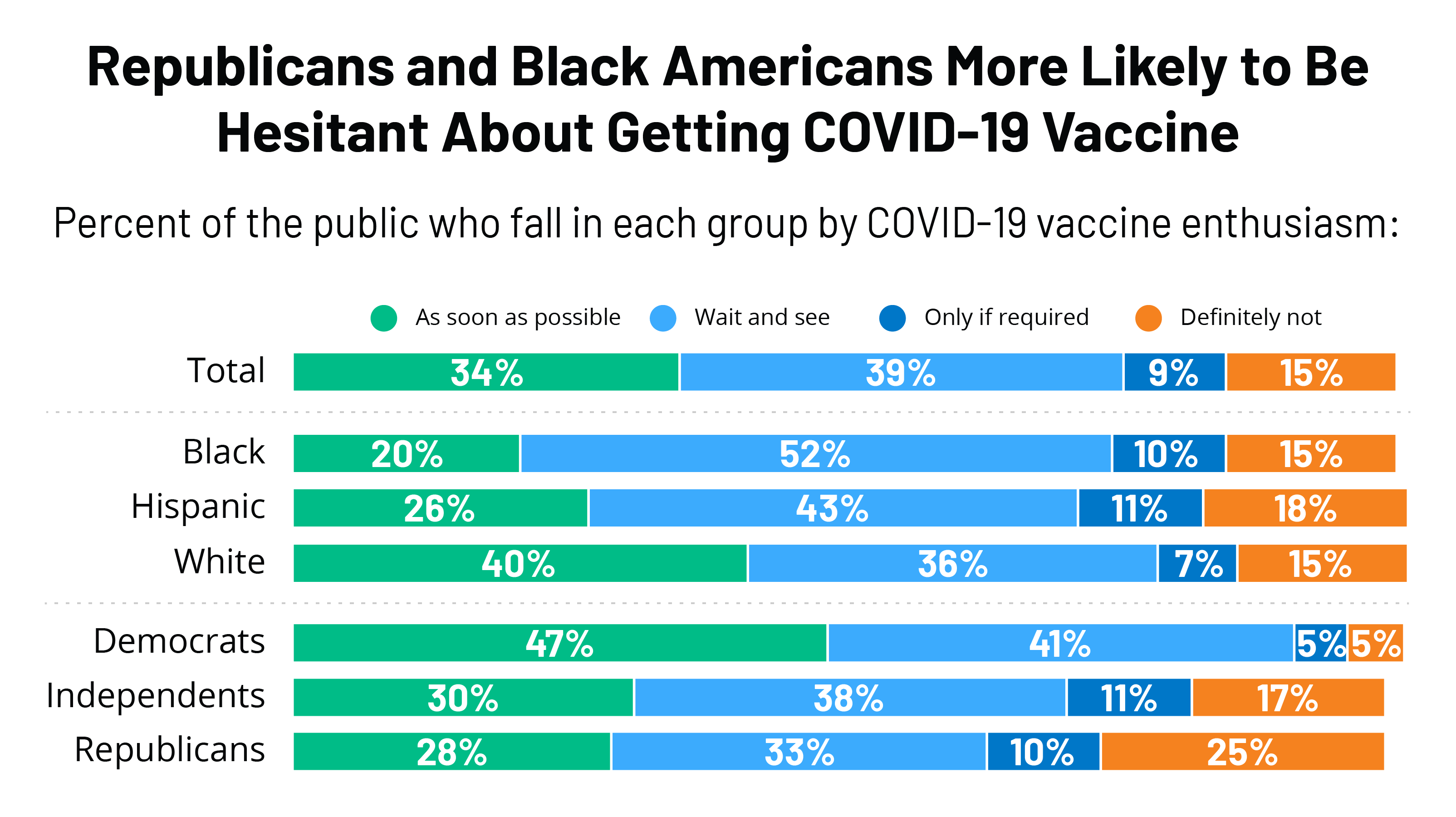
According to a June survey from the Kaiser Family Foundation 31 percent of unvaccinated adults said they would be more likely to get a vaccine if one of the vaccines currently authorized for emergency use received full FDA approval. Only people who have received all recommended doses of an FDA-authorized or WHO-listed COVID-19 vaccine. In fact its one of just seven vaccines that the WHO has approved for inclusion on the EUL.
The Sinopharm vaccine is produced by Beijing Bio-Institute of Biological Products Co Ltd subsidiary of China National Biotec Group CNBG. FDA employees who are career. Some polling data suggests that full FDA approval may boost vaccination rates among those yet to receive a COVID-19 shot.
Covid Vaccines For Kids Under 12 Expected Midwinter Fda Official Says

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021
Novavax Announces Further Delays For Regulatory Filings Of Covid 19 Vaccine Pmlive
Moderna Asks Fda For Adolescent Covid Vaccine Approval
Covid Vaccines What Full Fda Approval Means For You

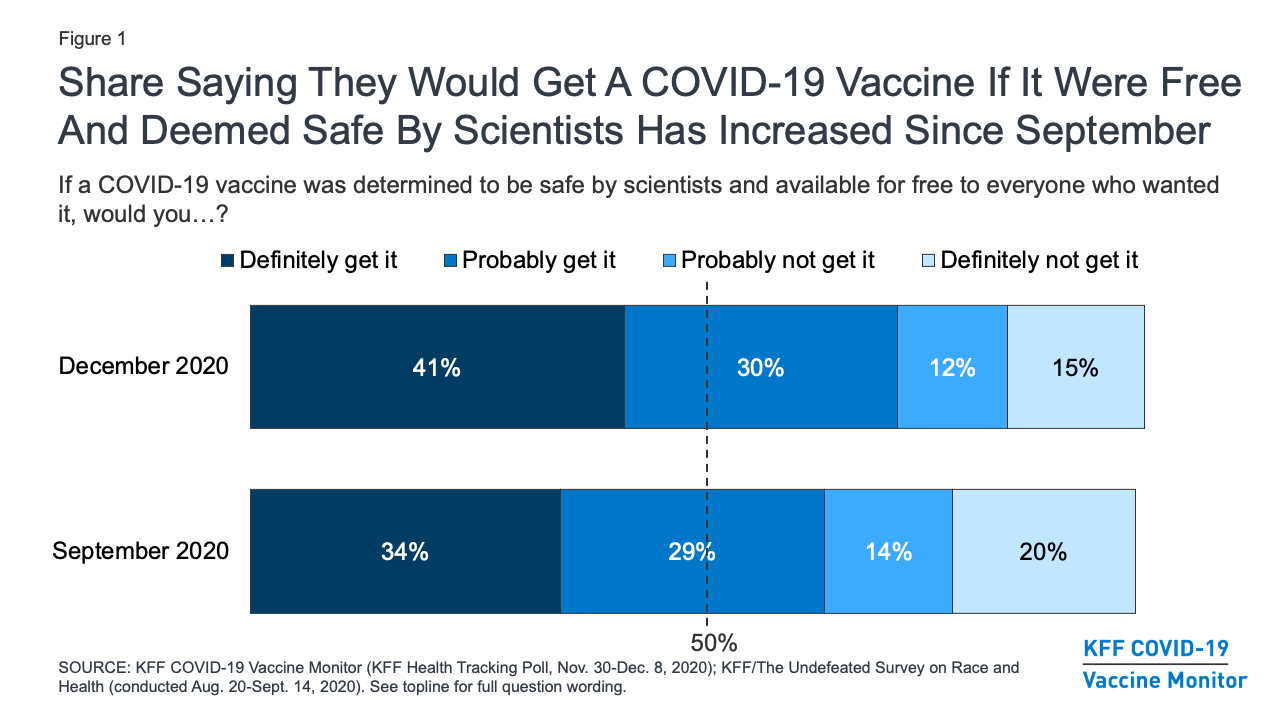
Kff Covid 19 Vaccine Monitor December 2020 Kff

Best Covid Vaccine To Get Comparing J J Pfizer Novavax And Moderna

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021

Fda Advisory Panel Endorses Pfizer Biontech Covid 19 Vaccine

Covid 19 Vaccine Trials 9 Things You Should Know Hackensack Meridian Health

Know Your Vaccines Vaccine Matrix Current Evidence Department Of Health Website

Know Your Vaccines Vaccine Matrix Current Evidence Department Of Health Website

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021
Covid Vaccine Moderna Applies For Full Fda Approval
What Full Fda Approval Could Change About Covid Vaccination

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021
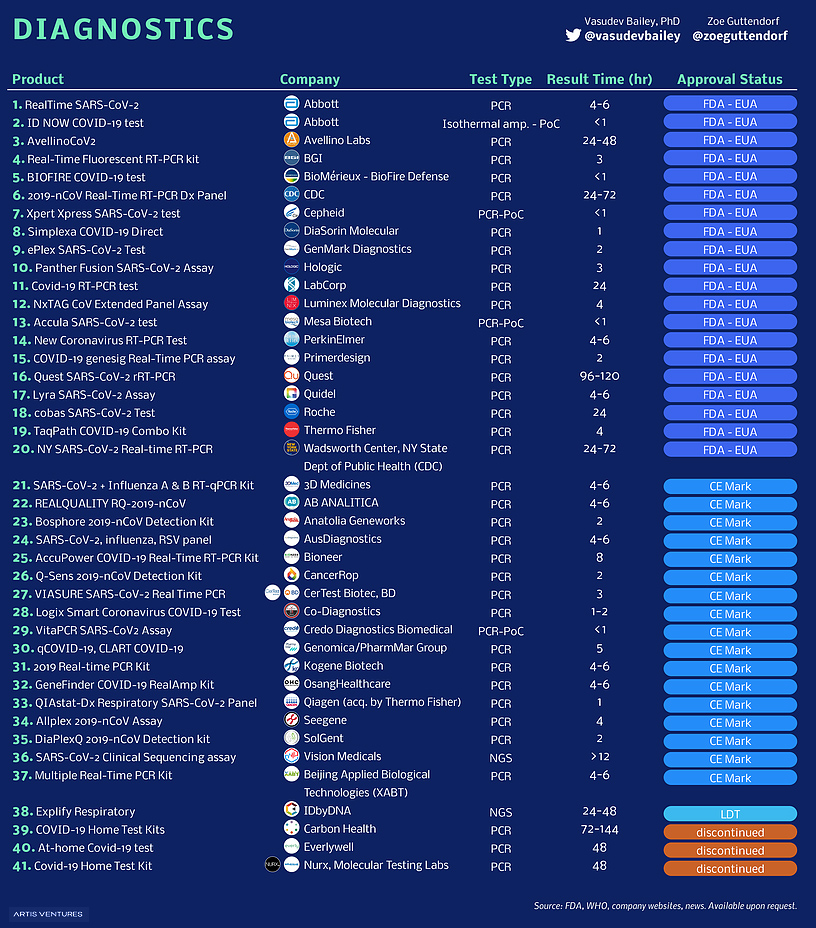
Every Vaccine And Treatment In Development For Covid 19 So Far