Health Canada reviews scientific evidence of a drug or vaccine including results from clinical trials to assess the products safety efficacy and quality before it can be sold in Canada. For COVID-19 vaccines Health Canada is using a fast-tracked process that allows manufacturers to submit data as it becomes available and for Health Canada experts to start the review process right away.
The Lead Covid 19 Vaccine Candidates Where We Are And What You Should Know Kilpatrick Townsend Stockton Llp Jdsupra
Review and approval of vaccines Scientific Review.
Fda approved vaccines canada. August 23 2021. Approved the distribution of mRNA coronavirus vaccines made by Pfizer and Moderna under the agencys emergency use. Pfizer and Moderna have both applied for full FDA approval for their jabs but it could be months away.
The Pfizer vaccine is currently approved in Canada for youth age 12 and up. Today the FDA announced the approval. None of the vaccines has been formally approved and only one COVID-19 therapeutic Gilead Sciences Incs GILD 107 Veklury has received full approval during the pandemic.
The FDA has officially approved the Pfizer COVID-19 vaccine. Heres where things stand. The Pentagon earlier this month said it plans to make Covid vaccinations.
104 Zeilen Coronavirus COVID-19 Update. 9 Zeilen Vaccines for human use. If a vaccine lot meets all requirements Health Canadas Biologic and Radiopharmaceutical Drugs Directorate issues a formal release letter to approve the sale of that lot in Canada.
To facilitate earlier access to COVID-19 drugs or vaccines Health Canada is prioritizing the review of these products while ensuring there is adequate evidence of safety efficacy and quality to merit. Vaccines will only be authorized once. Teams of Health Canada experts conduct a thorough and independent review of all vaccine data.
AstraZeneca COVID-19 Vaccine ChAdOx1-S recombinant solution for injection. In December 2020 the FDA. The lot release program enables Health Canada and the manufacturer to monitor consistency in the vaccine.
The two-dose vaccine which Pfizer developed in collaboration with German-based BioNTech was granted emergency use authorization by the FDA. Some universities and hospitals are expected to mandate inoculation once a vaccine is fully approved. The FDA formally approved the two-dose Pfizer-BioNTech COVID-19 vaccine for people 16 and over on Monday opening the door for more vaccine mandates across the country.
After months of anticipation Americans have a fully licensed COVID-19 vaccine. The FDA authorized the use under the emergency use authorization EUA for the Janssen COVID-19 vaccine of an additional batch of vaccine drug substance. Previously the regulatory agency had allowed the shots to be administered under an emergency use authorization a mechanism used.
Pfizers shot is the first COVID-19 vaccine to gain full FDA approval. About the vaccine how it works how it is given ingredients allergies possible side effects safety monitoring Pfizer-BioNTech COVID-19 vaccine AstraZenecaCOVISHIELD COVID-19 vaccine. July 1 2021 948 AM With the Delta coronavirus variant posing a serious risk to unvaccinated Americans some experts are calling for the FDA to fully approve.
While all three COVID vaccines have met FDAs strict standards for emergency use this FDA approval should give added confidence that. Pfizers two-dose Covid-19 vaccine has received full approval from the US Food and Drug Administration FDA - the first jab to be licensed in the nation. Canada becomes first country to approve Pfizer vaccine for children 12-15 May 6 2021.
Samples for Health Canada to test independently. Similarly vaccines that have been approved for use in this country by Health Canada like the AstraZeneca vaccine have not been approved in other countries like the US further. On Tuesday Modernas CEO said he expects to submit clinical data in early fall on vaccine trials in children aged six.
Authorized with terms and conditions. Vaccines for human use. The results of their own tests.
Interim Order Respecting the Importation Sale and Advertising of Drugs for Use in Relation to COVID-19. In most cases to sell each lot of vaccine in Canada the manufacturer must submit.
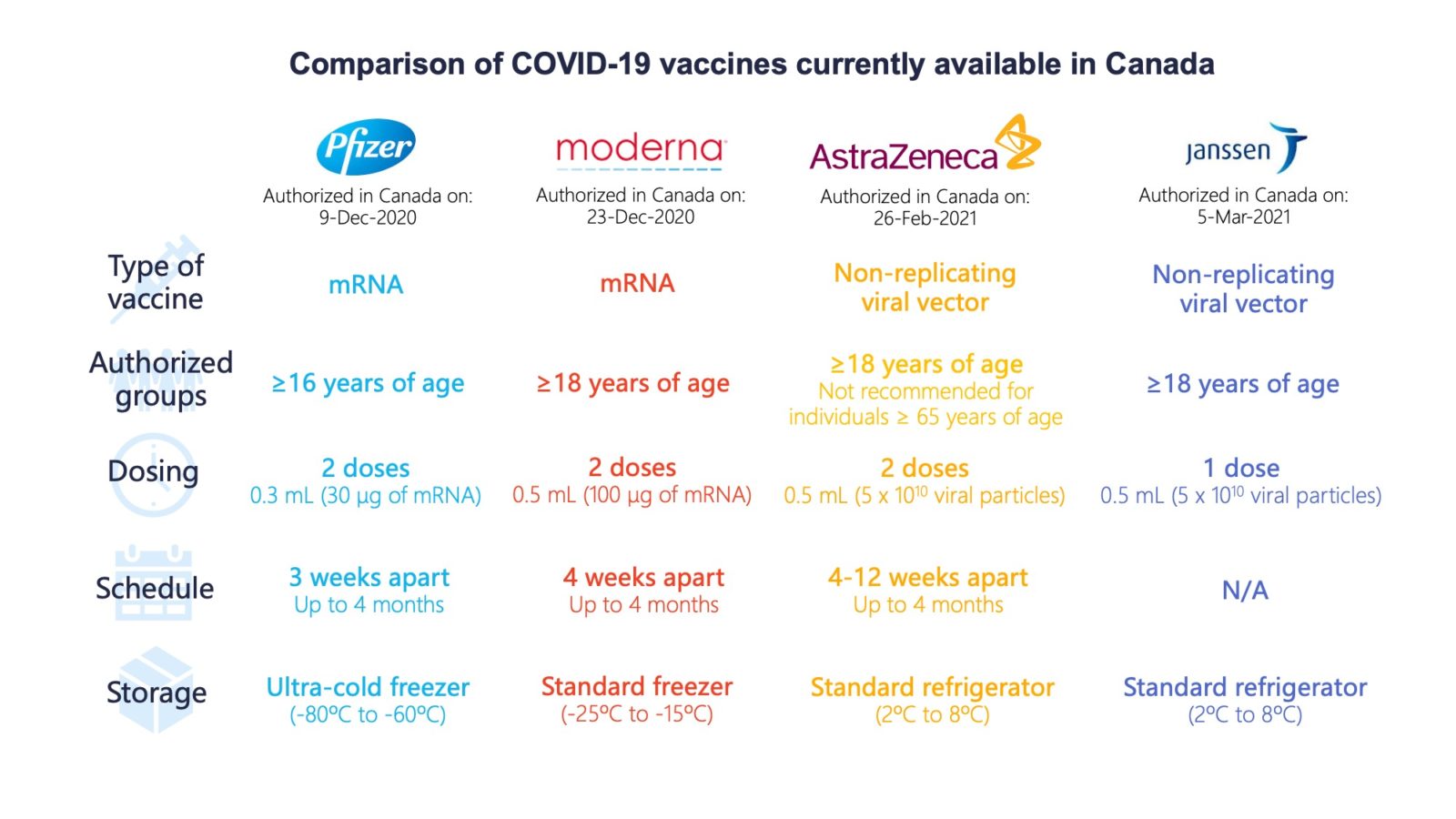
Comparing Vaccines Efficacy Safety And Side Effects Healthy Debate

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021
Canada Becomes First Country To Approve Pfizer Vaccine For Children Voice Of America English

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021

Lack Of Fda Covid Vaccine Approval Doesn T Matter Office For Science And Society Mcgill University

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021

Pfizer Delay Compounds Canada S Problems In Vaccine Campaign Bloomberg

Why Hasn T The Astrazeneca Vaccine Been Approved In The U S Yet Cgtn

Who S Next As Canada Uk And Us Approve Covid 19 Vaccines Coronavirus And Covid 19 Latest News About Covid 19 Dw 12 12 2020

Who S Next As Canada Uk And Us Approve Covid 19 Vaccines Coronavirus And Covid 19 Latest News About Covid 19 Dw 12 12 2020

Us Plans To Export 60 Million Astrazeneca Vaccine Doses News Dw 26 04 2021

Comparing Vaccines Efficacy Safety And Side Effects Healthy Debate

Medicago Vaccine S Rolling Submission To Be Reviewed By Health Canada

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021
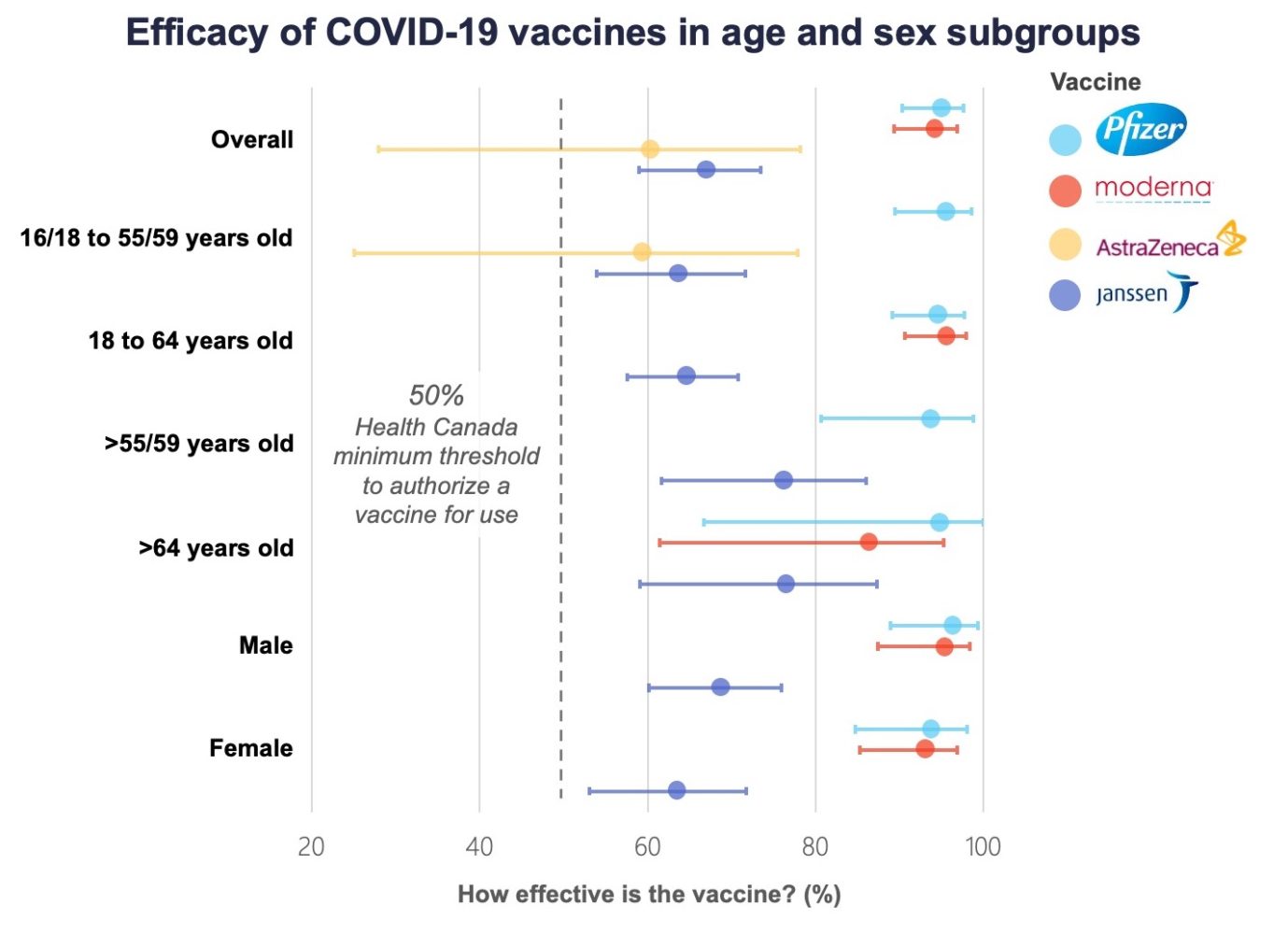
Comparing Vaccines Efficacy Safety And Side Effects Healthy Debate

Who S Next As Canada Uk And Us Approve Covid 19 Vaccines Coronavirus And Covid 19 Latest News About Covid 19 Dw 12 12 2020

Covid 19 Risks And Side Effects Of Vaccination Science In Depth Reporting On Science And Technology Dw 20 01 2021


