Sinopharm SARS-CoV-2 Vaccine Vero Cell. Soldiers assigned to Fort Bragg before being vaccinated.

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021
WHO also listed the PfizerBioNTech vaccine for emergency use on 31 December 2020.

Fda approved covid vaccines list. And COVID-19 vaccine Ad26COV2S developed by Janssen Johnson Johnson on 12 March 2021. Is encouraging people to get a Covid-19 shot but hasnt formally approved those vaccines. Pfizer BNT162b2COMIRNATY Tozinameran INN Moderna mRNA-1273.
The FDA recognizes that vaccines are key to ending the COVID-19 pandemic and is working as quickly as possible to review applications for full approval an FDA. Janssen Johnson. Do the authorized COVID-19 vaccines work.
Part of FDAs evaluation of an EUA request for a COVID-19 vaccine includes evaluation of the chemistry manufacturing and controls information for the vaccine. An FDA slideshow presentation regarding Covid vaccines last year accidentally displayed a long list of possible adverse reactions to the vaccine including myocarditis seizures and even death. Clinical data login required Paediatric investigation plan.
The review process could move. Vaxzevria previously COVID-19 Vaccine AstraZeneca Conditional marketing authorisation granted. Heres the Latest Currently no coronavirus vaccine is fully approved by the FDA but three were given emergency use authorization by the agency.
The Biden administration has stated that the federal government will not mandate vaccinations beyond the federal workforce but is increasingly urging state and local governments as well as businesses to consider. 9 Zeilen List of authorized drugs vaccines and expanded indications for COVID-19. In fact its one of just seven vaccines that the WHO has approved for inclusion on the EUL.
The slide showing the FDAs draft list of possible adverse event outcomes appeared briefly during a public meeting by the US Food and Drug Administrations Product Advisory Committee on Oct 22. EMA recommends COVID-19 Vaccine Moderna for authorisation in the EU. All three FDA-authorized vaccines prevent COVID-19 and serious health outcomes that COVID-19 can cause including hospitalization and deaths.
Two versions of AstraZeneca AZD1222. Equitable access to safe and effective vaccines is critical to ending the COVID-19 pandemic so it is hugely encouraging to see so many vaccines proving and going into development. The FDA has only granted emergency-use approval of the Pfizer Moderna and Johnson Johnson vaccines but the agency is expected to soon give full approval to Pfizer.
104 Zeilen The FDA expanded the emergency use authorization for the Pfizer-BioNTech COVID-19 Vaccine to include 12 15 year olds and issued an updated FDA COVID-19 Response At-A-Glance Summary. The three COVID-19 vaccines produced by Pfizer-BioNTech Moderna and Johnson Johnson have been granted authorization for emergency use by the FDA. All the ones mentioned below have products that have been approved.
The FDA expanded the emergency use authorization for the Pfizer-BioNTech COVID-19 Vaccine to include 12 15 year olds and issued an updated FDA COVID-19 Response At-A-Glance Summary. The Food and Drug Administration is working to approve the Pfizer - BioNTech Covid-19 vaccine on Monday The New York Times reported citing sources. Emergency Use AuthorizationEmergency Use Authorization EUA is an authorization issued for unregistered drugs and vaccines in a public health emergency.
The FDA has only granted emergency-use approval of the Pfizer Moderna and Johnson Johnson vaccines but the agency is expected to soon give full approval to Pfizer. 121 of the President of the Philippines authorizes the issuance of the EUAList of COVID-19 Vaccines Authorized by the FDAPfizer-BioNTech COVID-19 Vaccine. These are as follows.
Two AstraZenecaOxford COVID-19 vaccines on 15 February 2021 produced by AstraZeneca-SKBio Republic of Korea and the Serum Institute of India. Are COVID Vaccines FDA Approved. WHO is working tirelessly with partners to develop manufacture and deploy safe and effective vaccines.
Spikevax previously COVID-19 Vaccine Moderna Conditional marketing authorisation granted. The FDA Director General by virtue of the Executive Order No.
Novavax Announces Further Delays For Regulatory Filings Of Covid 19 Vaccine Pmlive

Fda Grants Full Approval To Pfizer S Covid Vaccine

Know Your Vaccines Vaccine Matrix Current Evidence Department Of Health Website
Moderna Asks Fda For Adolescent Covid Vaccine Approval
Fda Approves J J S Single Shot Covid Vaccine For Emergency Use

Why Two Vaccines Passed The Finishing Line In A Year And Others Didn T And A Month 12 Roundup Absolutely Maybe

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021

Covid Vaccine Side Effects What It S Like In Pfizer Moderna Trials

Lack Of Fda Covid Vaccine Approval Doesn T Matter Office For Science And Society Mcgill University

Best Covid Vaccine To Get Comparing J J Pfizer Novavax And Moderna

Fda Advisory Panel Endorses Pfizer Biontech Covid 19 Vaccine
Covid Vaccines What Full Fda Approval Means For You
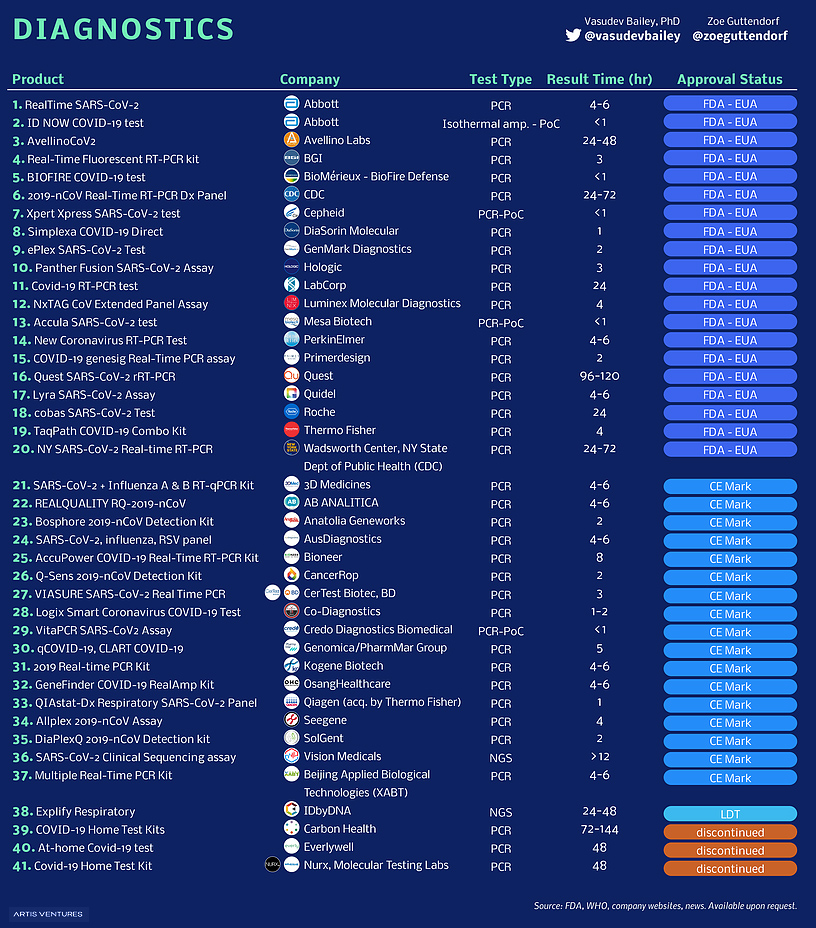
Every Vaccine And Treatment In Development For Covid 19 So Far

Know Your Vaccines Vaccine Matrix Current Evidence Department Of Health Website

Covid Vaccine Moderna Applies For Full Fda Approval
Covid Vaccines For Kids Under 12 Expected Midwinter Fda Official Says
What Full Fda Approval Could Change About Covid Vaccination

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021


